Czech Republic donated 30,000 doses of Moderna to Taiwan
Image : Web Screenshot (Photo by Yao Jiexiu)
Following the arrival of Taiwan's self-purchased 265,000 doses of AstraZeneca vaccine in Taiwan, the Czech Republic gave 30,000 doses of Moderna free to Taiwan in the afternoon of 29 August 2021.
Czech Republic's donated Moderna vaccines arrived in Taoyuan Terminal 2 at 5.49 pm by Turkish Airlines flight TK24.
Chen Shizhong, the commander of the Central Epidemic Command Center, said that the amount of 30,000 doses of vaccine is not particularly large and will be arranged for distribution according to the established original plan.
A total of 2.88 million people have checked the command centre for open willingness registration and said that the Czech government announced on 26 July that it will donate 30,000 doses of covi vaccine to Taiwan. The vaccines departed from Prague Airport in the early morning of 27 August and arrive at Taoyuan Airport in the afternoon of 29 August.
The new batch of Moderna vaccine is directly transported to the designated cold storage logistics centre for subsequent inspection and sealing operations. After the customs clearance procedures are completed, and the subjects listed in the covid vaccination plan will be provided for vaccination.
In addition, the command centre stated that at this moment when the global vaccine supply is in short supply, the Czech Republic lends a helping hand to provide this batch of covi vaccines, which will greatly help the domestic epidemic prevention and control. The command centre would like to express their sincere gratitude to the Czech government.
4 cases of breakthrough infections from abroad, one case vaccinated thrice with ZF2001
Image : Packaging of ZF2001. Courtesy of Anhui Zhifei Longcom Pharmaceutical Co., Ltd web-site.
The Central Epidemic Command Centre announced today (29 August 2021) that there are 10 new inbound cases of covid in Taiwan, of which 4 people have been fully vaccinated and determined to be breakthrough infections.
Luo Yijun, deputy leader of the Command Centre’s Medical Response Team, said that one of the women entered Taiwan from mainland China and was vaccinated with the local covi vaccine "ZF2001" in April and May 2021. ZF2001 is a recombinant protein subunit vaccine. The woman has completed 3 doses of ZF2001 within 6 months!
The command centre said that today there are 10 new inbound covid cases, 7 males and 3 females, aged from 10 to 60 years old, from Mainland China (Case 16071), Cambodia (Case 16074, 16075), The United States (Case 16076), Myanmar (Case 16088, 16089, 16090, 16091), Pakistan (Case 16092) and Vietnam (Case 16093) entered the country. The entry date was between 15 August and 28 August.
News (3)
Basic facts of ZF2001
Vaccine name: ZF2001
Generic name: Recombinant novel coronavirus vaccine (CHO Cell)
Trade name: ZIFIVAX
English name: Recombinant Novel Coronavirus Vaccine (CHO Cell)
Chinese Pinyin: Chongzu Xinxing Guanzhuang Bingdu Yimiao (CHO Cell)
Common Chinese name: 智飞疫苗
Developer: Anhui Zhifei Longcom Biopharmaceutical Co., Ltd.+ Institute of Microbiology, Chinese Academy of Sciences
Marketer: ZFSW The Biologics Company
【Package】
Vial. 0.5ml per bottle, 1 bottle per box.
【Validity Period】
Tentatively scheduled for 24 months.
Freezing is strictly prohibited. The vaccine can be stored and transport in the dark at 2~8°C.
ZF2001's Phase III clinical trials cover 29,000 participants in mainland China, Uzbekistan, Ecuador and Malaysia. It is reported Phase III clinical trial in mainland China has lately ended. It has Emergency Use Authorization (EUA) in mainland China and Uzbekistan.
Ingredients of ZF2001
This strain is a novel coronavirus (covi) spike glycoprotein receptor binding region NCP-RBD protein expressed by recombinant CHO cells. It is white, made by purification and adding aluminum hydroxide adjuvant. Main active ingredient is NCP-RBD protein in the receptor binding region of the new coronavirus spike glycoprotein. The adjuvant is aluminum hydroxide. Excipient ingredients include sodium chloride, disodium hydrogen phosphate, sodium dihydrogen phosphate, histidine.
Properties
This product should be a milky white suspension liquid, which can be stratified due to precipitation and easy to shake.
Vaccination target
People who are 18 years old and above who are susceptible to covi.
Function and Use
After being inoculated with this product, it can stimulate the body to produce immunity against covid and diseases caused by infection.
News (4)
The marketer does not guarantee ZF2001 has a total protective effect against covid
Based on the completed clinical research data on the immunogenicity and safety of the vaccine, Anhui Zhifei Longcom Biopharmaceutical Co., Ltd. suggested that ZF2001 has potential clinical application value, but because the human Phase III clinical study on the protective effect against covid has not been completed, vaccination of this product has not been determined to have a total protective effect against covid.
After vaccination, the body needs to produce neutralizing antibodies.
At a certain time, it is still necessary to take appropriate protective measures.
Specification
ZF2001 is in liquid injection form, 0.5ml per bottle, 0.5ml per human dose, containing NCP-RBD protein 25μg.
Immunization Procedure and Dosage
Usage: This product is recommended for intramuscular injection. Shake well before injection. Intramuscular injection into the deltoid muscle of the upper arm.
Dosage: In 0, 1, and 2 months, each person will inoculate 1 dose of ZF2001 each time, and 3 doses in total during the whole course.
News (5)
Adverse reactions after ZF2001 vaccination reported
ZF2001 has carried out Phases I and II clinical trials in 1,000 adults aged 18 years old and above, with a total of 182 subjects. They receive 3 doses of target dose immunizations, and follow-up observations on systemic safety are performed 0-7 days after each dose, 8-30 days.
After adopting the methods of active report of subjects and regular follow-up of investigators to collect adverse events, no seriousness related to this product was found.
Adverse events
According to the incidence recommended by the International Committee of Medical Science Organizations (CIOMS): very common (≥10%),
Common (1~10%, including 1%), occasionally (0.1~1%, including 0.1%), rare (0.01~0.1%, including 0.01%), it is rare (<0.01%), and the adverse reactions of this product are described as follows;
Systemic adverse reactions:
Common: Fever, headache, upper respiratory tract infection, cough, diarrhea, muscle pain (non-vaccination site).
Occasionally: Nasal congestion, oropharyngeal discomfort, esophageal pain, joint pain, dizziness, pruritus, vomiting, nausea, runny nose, limb pain, chest discomfort, irregular menstruation, abdominal distension, abdominal pain.
Local adverse reactions:
Very common: Pain, swelling, flushing, itch.
Common: Induration, rash.
In addition, it has been found in previous animal trials and studies of other coronavirus vaccines that the virus is re-infected after vaccination.
At that time, the disease caused by the virus aggravated. This vaccine has completed primate trials and Phases I and II human clinical trials.
The above phenomenon has not been observed in the test, and whether the above-mentioned safety problem also exists is still uncertain.
News (6)
Results of clinical trials of ZF2001 on subjects: 148 subjects up to 99.31% diagnosed positive turn negative
According to the randomized, double-blind, placebo-controlled Phases II clinical trials of this product in adults 18 years of age and older, among the samples from 148 subjects covered, the positive conversion rate of serum antibodies in these subjects was 99.31%, which means 2 out of 9 patients have no basic immune response. The protection of the population over the age of 18 may be insufficient.


Taboos
1. Those who are known to be allergic to any ingredient of the vaccine, including excipients.
2. People suffering from acute diseases, severe chronic diseases, acute episodes of chronic diseases, and fever.
3. Women during pregnancy.
4. People suffering from uncontrolled epilepsy and other progressive neurological diseases.
Precautions
Use with caution in the following situations: People with a history of seizures in family and individuals, people with chronic diseases, people with a history of epilepsy, allergies.
Shake well during use. If there are unshakable clots, foreign bodies, cracks in the vaccine bottle or unclear labels, neither can be used.
The vaccine should be used immediately after opening.
Drugs such as epinephrine should be prepared for emergency use in the event of a severe allergic reaction. The person receiving the injection should be observed at the scene for at least 30 minutes.
People who have abnormalities such as high fever and convulsions after the first injection, generally do not inject the second injection.
News (8)
ZF2001 reportedly protects subjects against covid up to 81.76%
China Chongqing Zhifei Biological Products Company announced on 27 August 2021 that the recombinant protein coronavirus vaccine developed by the company, ZF2001, has obtained results of the third phase of clinical trials, showing that the protection against covi is 81.76%.
Based on mainland Chinese news reports, Chongqing Zhifei Biological Products Co., Ltd. issued an announcement stating that the company's wholly-owned subsidiary Anhui Zhifei Longcom Biopharmaceutical Co., Ltd. and the Institute of Microbiology, Chinese Academy of Sciences jointly developed the new recombinant covi vaccine (CHO cell) and obtain key data from Phase III clinical trials. This is mainland China's first recombinant protein vaccine to publish clinical Phase III data.
According to the announcement, since 12 December 2020, the vaccine has successively carried out international multi-centre Phase III clinical trials in Hunan Province, Uzbekistan, Indonesia, Pakistan and Ecuador. 28,500 people were actually enrolled, including 14,251 in the vaccine group and 14,249 in placebo cases.
The announcement stated that a total of 221 cases of the primary endpoint after the whole course of vaccination were monitored, and the protective efficacy for any severity of covid was 81.76%, which met the vaccine effectiveness standards required by the World Health Organization. Among them, the protection effect for severe covid and above cases and death cases is 100%.
Media reports on ZF2001's protection rate against covi strains differ. According to Lianhe Zaobao, preliminary genotyping analysis results showed that the vaccine's protective efficacy against Alpha variant strains was 92.93%, and the protective efficacy against Delta variant strain (Covi72) was 77.54%. The Straits Times rates it 78% effective against Covi72.
According to reports, ZF2001 was put into emergency use in China in March 2021. It is the fourth coronavirus vaccine approved for emergency use in China and the first recombinant covi subunit protein vaccine approved for clinical use in the world.
Phase III clinical trial of ZF2001 in Uzbekistan is expected to complete by December 2021 while Malaysia has yet to carry out Phase III clinical trial of the vaccine.
News (9)
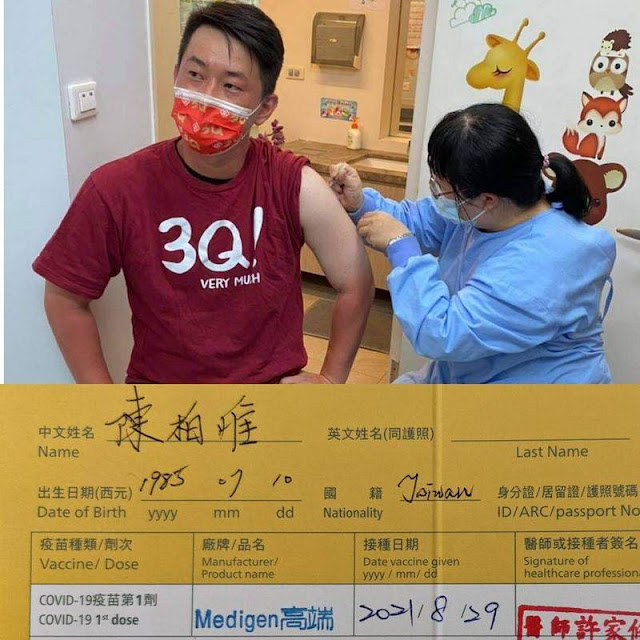
The vaccination experience is "excellent"! Chen Baiwei exposes his small yellow card Medigen vaccine "experiences''
Image : Chen Baiwei went to a local clinic to get a Medigen vaccine today (29) and revealed that the injection experience was excellent. He also said with a smile that mosquitoes might bite harder than the vaccine. (The picture is taken from Chen Baiwei's Facebook)
Medigen MVC COVID-19 Vaccine (MVC-COV1901) was launched on 23 August, and Chen Baiwei, a legislator of the DPP Taichung, also went to the local clinic on 29 August 2021 and revealed that the MVC injection experience is excellent, "Good, I also said with a smile that mosquitoes may bite harder than vaccines."
Chen Baiwei posted on Facebook this afternoon that he had finished his first dose of MVC-COV1901. "About a few tips, it is said that the cost is very high and the injection experience is excellent. The mosquitoes may bite harder than the vaccine, and you don’t need to rub them. The atmosphere at the vaccination station was different. Before, I used to comfort and explain. Everyone was chatting and talking here. The atmosphere was very warm. It seemed that no one took painkillers before coming here."
Chen Baiwei said that, like the World Health Organization, we encourage everyone to vaccinate when it’s your turn. If you have any doubts or specify a vaccine, you can also go to the clinic to ask about your medical history and condition and give advice. "We will win first together. After the war, the next step is to use vaccines to maintain success. I pray that the epidemic in Taiwan will be restored as soon as possible. Taiwan, come on."
Chen Baiwei also mentioned that the legislators are working hard to understand and coordinate the doubts about student age and vaccines, and hope that the coverage rate will be better.
News (10)
Study: Fully vaccinated carry 251 times the normal load of covi, may be super spreaders
Reporter : Andrew White, National File
A study by the Oxford University Clinical Research Group published on 10 August 2021 in The Lancet found that fully vaccinated people carry 251 times the viral load of coronavirus as compared to those who have not received one of the controversial vaccines. “Viral loads of breakthrough Delta variant infection cases were 251 times higher than those of cases infected with old strains detected between March-April 2020,” reads the study.
The vaccines seem to allow vaccinated individuals to carry unusually high viral loads without becoming sick, transforming them into super spreaders who experience symptoms later on, as reported by Daily Veracity:
The study focused on healthcare workers who were unable to leave the hospital for two weeks. The study showed that fully vaccinated workers, about two months after injection, carried and transmitted the virus to their vaccinated colleagues after infection.
They also passed the virus to unvaccinated people, including their patients. The vaccine used in the study was the Oxford/AstraZeneca (AZD1222) vaccine.
In reaction to the study, Congressman Paul Gosar (R-AZ), tweeted “as we have said—side effects and efficacy are measured in years. Not weeks and months. Another study that should cause all reasonable people to pause.”
The Food and Drug Administration (FDA) on Monday granted full approval for the Pfizer-BioNTech COVID-19 vaccine, opening the door for vaccine mandates across the US. This comes just days after the Director of the Center for Disease Control (CDC) Rochelle Walensky admitted that COVID-19 “vaccine effectiveness against SARS-CoV-2 is waning,” adding that those who were “vaccinated early” are at an “increased risk of severe disease.”
SEC filings have shown that Moderna has made $12 billion as the government and major institutions push for forced vaccinations, as was previously reported by National File. Stéphane Bancel, the Chief Executive Officer of Moderna said they are “looking forward towards our vision of a single dose annual booster that provides protection against COVID-19, flu and RSV for adults. I look forward to the start of our Phase 3 trial for CMV this year and to clinical proof of concept data in the coming quarters from our therapeutics pipeline. We believe this is just the beginning.”
Ref: https://nationalfile.com/study-fully-vaccinated-carry-251-times-the-normal-viral-load-of-covid-19-may-be-super-spreaders/









No comments:
Post a Comment